xMRI Lab (Nagel)
Welcome to the research group Metabolic and Functional MR Imaging!
We always offer exciting Bachelor, Master and PhD topics. If you are interested, please send an email to armin.nagel@uk-erlangen.de.
The research group develops MR measurement techniques for the characterization of metabolic and functional processes. The focus of our research is in the area of ultra-high field (7 Tesla) and so-called X-nucleus MRI. “X” here stands for any atomic nucleus with nuclear spin, except 1H.
1. Imaging of ions (Na+, K+, Cl–)
Sodium (Na+), potassium (K+), and chloride (Cl–) ions play a vital role in many cellular processes such as the excitability of neurons and muscle cells. MRI of these nuclei – often denoted as X-nuclei MRI – is a promising approach to non-invasively examine cell viability.
Over the past decades, 23Na MRI has become a widely used technique to noninvasively determine the total tissue sodium concentration. It is a valuable tool in biomedical research despite its challenges that include low signal-to-noise-ratio (SNR) and fast signal decay [1,2]. Imaging of chloride [3] and potassium [4,5,6] is even more challenging due to the even lower signal intensity, which makes the use of ultra-high field strengths (B0 ≥ 7 T) indispensable.
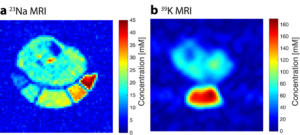
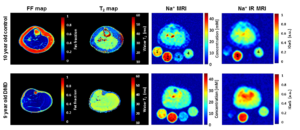
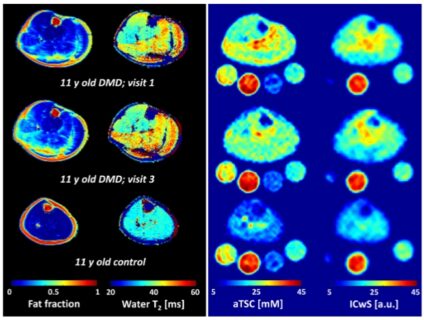
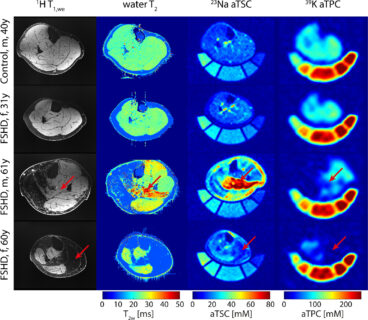
Figure 1 shows exemplary sodium and potassium concentration maps of healthy lower leg muscle tissue acquired with a dual-tuned 23Na/39K calf coil at 7 T [6]. Figure 2 shows sodium images of a healthy volunteer and a patient with Duchenne muscular dystrophy [7]. Elevated muscular sodium signal intensities were regularly observed in patients with Duchenne muscular dystrophy (DMD) compared with controls, and were present even in absence of fatty degenerative changes and water T2 increases [7]. This Na+ overload might contribute to the disease progression [8]. Thus, 23Na MRI may be considered as a potential marker to characterize dystrophic muscle tissue at an early stage.
Probing the molecular environment of sodium and potassium ions using 23Na and 39K MRI
One approach to analyze the molecular environment is to examine the existence of multiple quantum coherences (MQC) as they are directly linked to the sodium ions’ molecular environment and the corresponding quadrupolar interactions [9]. MQC describe superpositions between nuclear energy levels with a difference in nuclear quantum number of Δm > 1 that can be induced in a system of nuclei possessing a nuclear spin I ≥ 1. Spin-3/2 nuclei as 23Na and 39K exhibit four nuclear Zeeman levels, therefore double quantum coherences (Δm = 2) and triple quantum coherences (Δm = 3) can be generated. In contrast to single quantum coherences, MQC are not directly MR observable. Instead, so-called multiple quantum filters (MQF) have to be applied to detect them. Triple quantum filtered imaging (TQF) offers the possibility to detect signal of ions located within restricted motional regimes and has been shown to provide weighting towards intracellular space. Double quantum filtered MRI with magic angle excitation (DQF-MA) can be used to selectively detect ions located within anisotropic structures such as muscle fibers. However, all these techniques suffer from low SNR and are prone to magnetic field inhomogeneities [10,11].
In this project, imaging techniques based on multiple quantum filtration are developed and applied both to healthy subjects and patients with muscular pathologies. The aim of this project is to probe the molecular environments (e.g. intra vs. extracellular) of sodium and potassium ions by making use of their quadrupolar interactions.
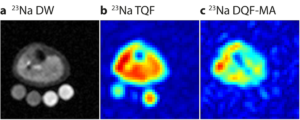
In Figure 3, a spin density weighted (DW) 23Na image of human lower leg is compared to a triple quantum filtered (TQF) and a double quantum filtered with magic angle excitation (DQF-MA) 23Na image [11].
2. Ultra-High Field (7 Tesla) MRI
One of the main challenges in magnet resonance imaging (MRI) is the limited signal-to-noise ratio (SNR). The University Hospital Erlangen is one of the few sites hosting a clinically approved Ultra High Field 7 Tesla MRI system. The increased field strength compared to conventional systems allows for a greatly improved SNR so that image resolutions of a few 100 micrometers can be reached.
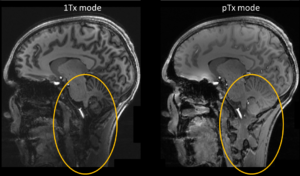
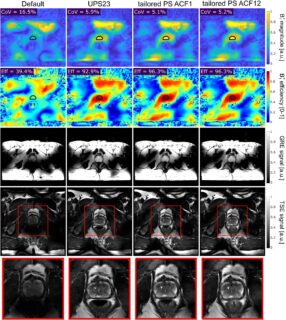
As the wavelength of the transmit field becomes of the same size as the human head at 7 Tesla, one must face the challenge of minimizing the arising inhomogeneous image brightness (Fig. 4, left). Moreover, the electric field may become inhomogeneous potentially entailing hot spots of the specific absorption rate (SAR), which must be taken into account in SAR calculations.
In order to achieve a homogeneous transmit field, we shape the radio frequency (RF) excitation field using eight different transmission coils, which transmit in parallel (parallel Transmit, pTx) [12]. For this, the transmission coils and the gradient coils are driven simultaneously but independently with individual voltage curves and pulse shapes. The basic underlying calculation approach is called ‘Transmit-SENSE’, in analogy to the established ‘SENSE’ algorithm using receive coil sensitivity profiles. During excitation, gradients are applied to influence the static magnetic field using the concept of ‘transmit k-space’. The optimal interaction of k-space trajectory and RF pulse shapes of all transmit coils is expressed as a minimization problem to be solved patient-specifically during an examination and as fast as possible to make it feasible in clinical routine.
Using this approach, images with little brightness inhomogeneity and low SAR exposure can be achieved (Fig. 5, right) [13].
[1] Huhn K, Engelhorn T, Linker RA, Nagel AM.
Potential of Sodium MRI as a Biomarker for Neurodegeneration and Neuroinflammation in Multiple Sclerosis.
Front Neurol 2019; 10(84).
[2] Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, Schmitter S, Speck O, Straub S, Zaiss M.
Pros and cons of ultra-high-field MRI/MRS for human application.
Prog Nucl Mag Res Sp 2018; 109: 1-50.
[3] Nagel AM, Lehmann-Horn F, Weber MA, Jurkat-Rott K, Wolf MB, Radbruch A, Umathum R, Semmler W.
In vivo 35Cl MR imaging in humans: a feasibility study.
Radiology. 2014 May;271(2):585-95.
[4] Umathum R, Rösler MB, Nagel AM.
In vivo 39K MR imaging of human muscle and brain.
Radiology. 2013 Nov;269(2):569-76.
[5] Nagel AM, Umathum R, Rösler MB, Ladd ME, Litvak I, Gor’kov PL, Brey WW, Schepkin VD.
39K and 23Na relaxation times and MRI of rat head at 21.1 T.
NMR Biomed. 2016 Jun;29(6):759-66.
[6] Gast LV, Mueller M, Hensel B, Uder M, Nagel AM.
Combined 23Na/39K MRI for the quantification of Na+ and K+ concentrations in human skeletal muscle at 7 T.
Proc Intl Soc Mag Reson Med 27 2019.
[7] Gerhalter T, Gast LV, Marty B, Martin J, Trollmann R, Schussler S, Roemer F, Laun FB, Uder M, Schroder R, Carlier PG, Nagel AM.
23Na MRI Depicts Early Changes in Ion Homeostasis in Skeletal Muscle Tissue of Patients With Duchenne Muscular Dystrophy.
J Magn Reson Imaging 2019; https://doi.org/10.1002/jmri.26681
[8] Lehmann-Horn F, Weber MA, Nagel AM, Meinck HM, Breitenbach S, Scharrer J, Jurkat-Rott K.
Rationale for treating oedema in Duchenne muscular dystrophy with eplerenone.
Acta Myol 2012; 31(1): 31-39.
[9] Navon G, Shinar H, Eliav U, Seo Y.
Multiquantum filters and order in tissues.
NMR Biomed. 2001;14(2):112-132.
[10] Matthies C, Nagel AM, Schad LR, Bachert P.
Reduction of B(0) inhomogeneity effects in triple-quantum-filtered sodium imaging.
J Magn Reson. 2010;202(2):239-244.
[11] Gast LV, Gerhalter T, Hensel B, Uder M, Nagel AM.
Double quantum filtered 23Na MRI with magic angle excitation of human skeletal muscle in the presence of B0 and B1 inhomogeneities.
NMR Biomed. 2018; e4010.
[12] Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, Schmitter S, Speck O, Straub S, Zaiss M.
Pros and cons of ultra-high-field MRI/MRS for human application.
Prog Nucl Mag Res Sp 2018; 109: 1-50.
[13] Herrler J, Liebig P, Gumbrecht R, Ritter D, Schmitter S, Maier A, Schmidt M, Uder M, Doerfler A, Nagel AM.
Fast online-customized (FOCUS) parallel transmission pulses: A combination of universal pulses and individual optimization.
Magn Reson Med 2021;85(6):3140-3153.