qMRI Lab (Laun)
Welcome to the qMRI lab!
The “q” in qMRI stands for quantitative as we work on quantiative MRI methods.
It moreover stands for q-space. The q-space is the space in which we acquire data in diffusion-weighted MRI (DWI), which is one of our research foci. With DWI, we measure water diffusion in tissue in vivo. The measurement of diffusion allows us drawing conclusions about tissue structure or tissue integrity and is used clinically, for example, in stroke diagnostics and in the diagnosis of prostate carcinoma. Our research focuses on the measurement of anisotropic diffusion (diffusion tensor imaging), non-Gaussian diffusion processes (e.g., kurtosis imaging, IVIM imaging), determination of tissue microstructure (e.g., diffusion pore imaging), and the development of high-gradient methods (e.g., dedicated breast gradients, G > 1 T/m). To enable quantitative evaluation, suitable validation, reference, and calibration objects, so-called phantoms, are also being developed.
Another focus is on quantitative susceptibility mapping (QSM). Different biological tissues differ in their magnetic susceptibilities, which can be measured and quantified using suitable MRI techniques. This technique can be used, for example, to differentiate between calcification and hemorrhage.
Moreover, we work on quantifying tissue stiffness and further elastography properties within the CRC 1540 “Exploring brain meachnics” using magnetic resonance elastography (MRE). MRE allows one e.g. to assess mechanical properties of the developing brain and of brain malformations.
We always offer exciting Bachelor, Master, and PhD topics. If you are interested, please send an email to frederik.laun@uk-erlangen.de.
Project group “MR Susceptibility Imaging” (headed by Jannis Hanspach)
Magnetic susceptibility has long been regarded as a nuisance factor in magnetic resonance imaging (MRI) that leads to artifacts. Using modern techniques such as susceptibility weighted imaging (SWI) or quantitative susceptibility mapping (QSM), the effect of magnetic susceptibility on MR phase maps can be used to generate useful contrasts and to calculate the distribution of magnetic susceptibility. Changes in magnetic susceptibility allow indirect conclusions regarding the spatial distribution of iron, myelin or the calcium content in the brain, which can be altered in neurodegenerative diseases such as Parkinson’s disease. In the human body outside the brain, sources of susceptibility such as calcifications, bleedings or foreign bodies can be easily visualized using these methods.
We are working on enabling the use of QSM and SWI in various anatomical regions, such as the brain, prostate, breast and kidneys, using new deep learning reconstruction methods and improving image quality. In addition, we support the use of QSM and SWI in patient studies in which we determine the distribution of magnetic susceptibility in cooperation with clinical partners. The aim here is to gain insights into the value of these techniques and potentially enable better diagnostics. Among other things, we are focusing on Parkinson’s disease, multiple system atrophy and prostate cancer patients.
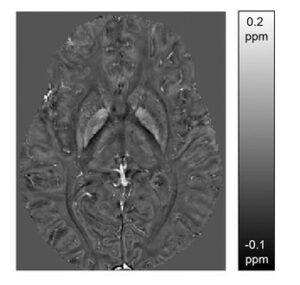
Quantitative susceptibility map of the brain of a healthy subject at the level of the basal ganglia. The susceptibility map was reconstructed from the phase map of a gradient echo sequence (7T), shows a unique contrast and provides conclusions about the spatial distribution of iron, myelin and calcium.
Project Group “Magnetic Resonance Elastography” (MRE, headed by Guillaume Flé)
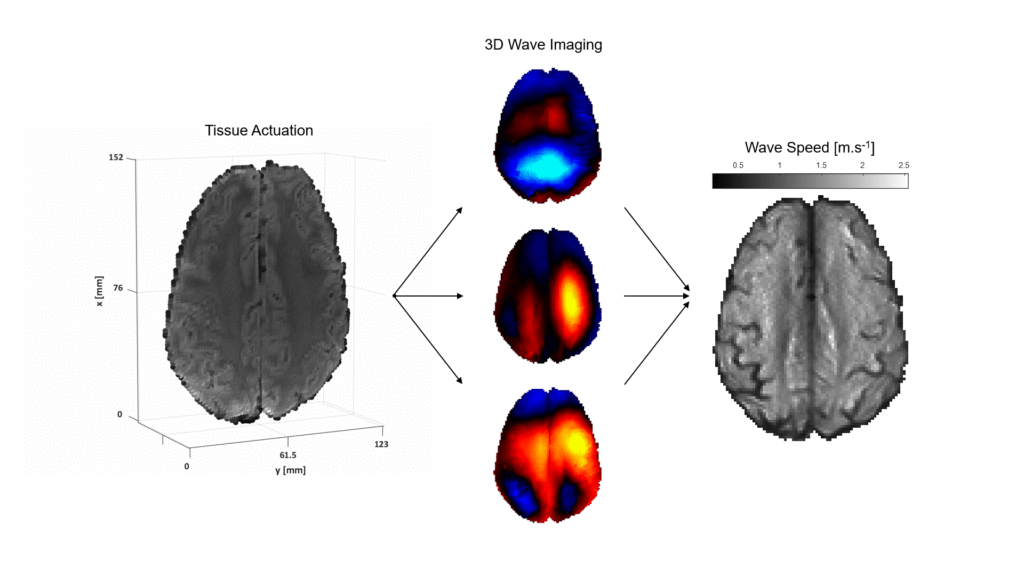
From individual cells to entire body regions, mechanical properties of tissue are dynamic markers of health condition and determinative actors in various biological processes. In medical settings, magnetic resonance elastography imaging (MRE) provides a unique means for the non-invasive assessment of organ stiffness using continuum mechanics principles. MRE induces weak time-harmonic vibrations in the scanned area using surface actuators positioned against the patient’s body in the MRI scanner. Propagation of the vibrations in the form of elastic waves is monitored by a specialised phase-contrast MR sequence that captures snapshots of the waves at consecutive instances of the harmonic actuation cycle. Processing of the wave images by a dedicated mechanical model produces maps of the distributed model parameters, often expressed in pairs such as wave propagation speed and attenuation coefficient or stiffness and viscosity related quantities.
The MR Elastography group investigates the biomechanics of the healthy and pathological human brain, with an ultimate focus on patients with epilepsy. In addition, the aim of our research is to contribute ex vivo data for comparison with different characterization methods.
We are part of the CRC1540 Exploring Brain Mechanics’ Y Project, headed by Arnd Dörfler, Frederik Laun, Jing Guo, and Ingolf Sack (https://www.crc1540-ebm.research.fau.eu).
Project: Investigation of pulsation-artifact-reducing techniques in diffusion imaging of the liver (DFG project number 446875476)
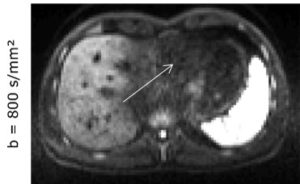
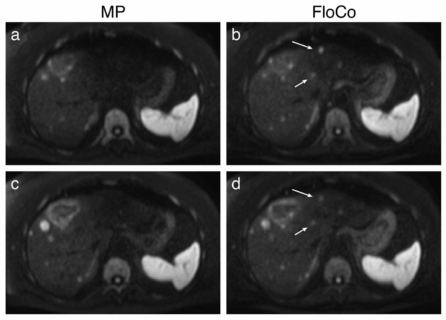
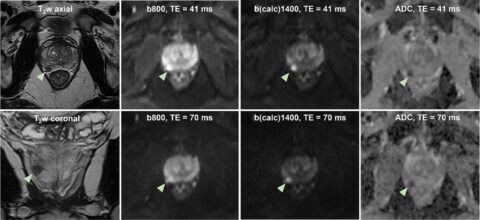
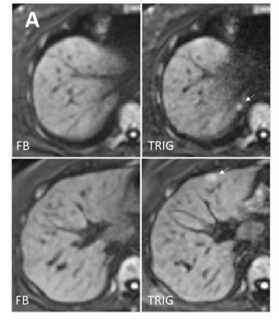
In magnetic resonance imaging of the liver, diffusion-weighted imaging (DWI) often shows signal loss in the left lobe of the liver, which is caused by cardiac pulsation. The goal of this project is to improve the image quality of liver DWI. For this purpose, this artifact shall be reduced while at the same time the blood signal must be suppressed. For this purpose, a “mixed” protocol will be used, the usability of which has been demonstrated in a recent own volunteer study. It includes a conventional bipolar diffusion coding at a small b-value (50 s/mm²) and a flow-compensated diffusion coding at a high b-value (800 s/mm²). This protocol will be used in a prospective patient study in which patients with known or suspected liver lesions will be included. One of the most important parameters that will be evaluated is the visibility of lesions in the left lobe of the liver. To further improve image quality, advanced data processing techniques will be developed and evaluated. Conventional approaches, e.g., outlier detections, will be pursued. In addition, “convolutional neural networks” will be trained to generate high-quality images from the set of acquired diffusion-weighted images.
This project is conducted in cooperation with clinical partner Marc Saake.
Project: Diffusion-weighted magnetic resonance imaging with flow-compensated diffusion weightings and tri-exponential IVIM imaging (DFG project number 437119659)
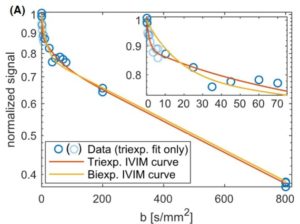
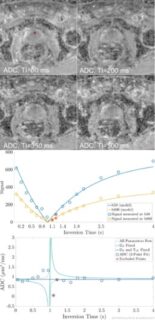
The Intravoxel Incoherent Motion (IVIM) model of Denis le Bihan et al. relates the observed sharp signal drop in diffusion-weighted magnetic resonance imaging at small b values to the presence of a compartment containing blood as a flowing fluid. The IVIM model is attractive because it allows studing perfusion parameters in native examinations, that is, without the administration of contrast agents. Increasing discussions about deposition of gadolinium-containing contrast agents in the human brain have further contributed to its attractiveness. This project builds on two recent research findings. First, the IVIM effect disappears when short flow-compensated diffusion weightings are used, but reappears when longer flow-compensated diffusion weightings are used. The use of the IVIM model by Denis le Bihan et al. makes it possible to link this experimental observation to parameters such as blood flow correlation time τ and vessel segment length l. These parameters are of great importance in the context of angiogenesis in tumor diseases. Second, recent work showed that the often used bi-exponential IVIM model is no longer valid at very small b values and a tri-exponential model seems to be more appropriate. The cause of the tri-exponential behavior is currently unclear, but an attractive interpretation would be, for example, to assign the measured two flow compartments to venous and arterial blood. This project aims to understand the mechanisms that produce contrast in flow-compensated and tri-exponential IVIM imaging. Experimentally, this will be achieved by performing flow-compensated and tri-exponential IVIM measurements with varying echo time (TE) at varying field strength (B0). This should help to identify the contributions of different compartments (such as venous, arterial, primary urine in the kidney). For this purpose, the property that the transverse relaxation time of these compartments is different and furthermore depends on B0 will be used. Since these IVIM measurements are experimentally challenging, part of the project is focused on optimizing the measurement protocol. The planned experiments will be performed on clinical scanners in healthy volunteers.
Project: On the diagnostic value of stimulated-echo MRI diffusion tensor imaging of the female breast. (Marohn-Stiftung)
Stimulated echo diffusion imaging (STE-DWI) is a new technique for examining the female breast using MRI. It does not require a contrast agent. The findings of a publication (8 patients) in 2017 suggest that this technique has the potential to overcome limitations of conventional MRI diffusion imaging, which is already widely used. Limitations include often poor quality of fat saturation and ambiguous separation of malignant and benign lesions by the measured quantitative diffusion coefficients. In this project, a clinical study will be performed in close cooperation between physicians and physicists to evaluate the clinical value of this new approach with respect to clinical use and for early detection programs. In the first phase, the technique and a protocol for the clinical study will be developed (physics part), which will be performed in the second phase (radiology part). Seventy female patients will be studied. The quality of the diffusion-weighted images and the discriminatory power of the quantitative diffusion coefficients will be evaluated for this purpose.
This project is carried out in cooperation with the clinical partners Evelyn Wenkel, Sabine Ohlmeyer, and Sebastian Bickelhaupt.